
by Gordon Post | May 10, 2024 | Uncategorized
Acids play a pivotal role in agriculture and food production, serving a variety of functions that range from conditioning soil to preserving food products. These different acids can significantly enhance efficiency and productivity in farming and food-processing...
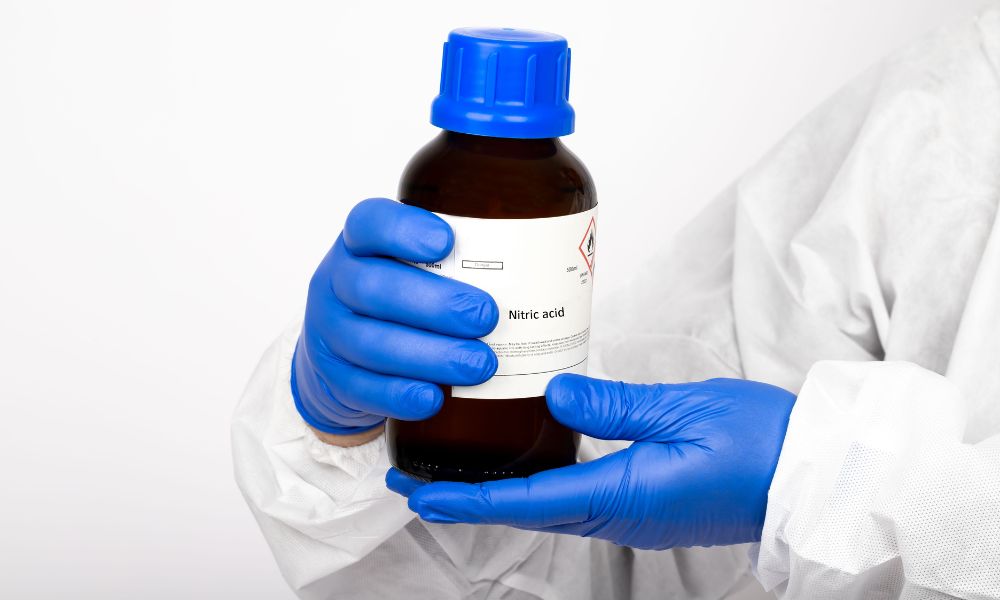
by Gordon Post | May 9, 2024 | Uncategorized
In the vast chemical industry, nitric acid stands out as a crucial reagent with a wide range of uses and applications. From fertilizers to chemical research, the versatile functions nitric acid provides make it a backbone of modern chemical processes. However, this...

by Gordon Post | May 3, 2024 | Uncategorized
Caffeine is infamous for fueling us on long workdays and kick-starting our morning rituals. How does it exist in the beverages we consume? The chemical process of isolating and sublimating caffeine is a fascinating topic of scientific ingenuity and chemistry. For...

by Gordon Post | Apr 23, 2024 | Uncategorized
Water plays a crucial role in laboratory procedures, acting as both a solvent and a reactant in many experiments. Different experiments demand various types of water, ranging from tap water to ultrapure water, each suited for specific applications. The purity levels...

by Gordon Post | Apr 19, 2024 | Uncategorized
Pharmaceutical compounding is a complex process requiring precision and adherence to stringent safety protocols at every stage. Within this domain, powder containment hoods safeguard workers’ well-being and the purity of the compounds they handle. These specialized...

by Gordon Post | Apr 11, 2024 | Uncategorized
High school chemistry labs are places of scientific discovery and hands-on learning. These environments are where students get to witness the magic of chemical reactions and understand the importance of chemistry in daily applications. Understanding which chemicals...







