Your cart is currently empty!
Author: Kate Romeo
-

Why Material Safety Data Sheets Are Vital in a Lab
Working in a lab comes with unique risks and responsibilities. Understanding and managing these risks is important for safety and efficiency. This guide will go over why material safety data sheets are vital in a lab.
What Are Material Safety Data Sheets?
Material safety data sheets (MSDS) provide detailed information about chemicals in your lab. They cover everything from potential hazards to safe handling procedures. Labs use MSDS to ensure everyone knows the risks associated with each chemical and how to manage them.
Compliance With Regulations
Material safety data sheets are vital for working in labs because lab environments must comply with various safety regulations. MSDS help labs meet these legal requirements. The United Nations Economic Commission for Europe (UNECE) established the regulations for MSDS through the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). The United States and European Union have adopted this system.
Lab owners and managers must comply with these regulations by providing updated and accurate MSDS for all hazardous chemicals in the workplace. They must also train employees on how to read and understand the sheets to ensure the safe handling of chemicals.
Failure to comply can result in severe penalties, such as fines, and put everyone’s safety at risk. In general, fines for non-compliance with SDS regulations can range from hundreds to thousands of dollars, depending on the severity of the violation. Additionally, not complying with safety regulations can result in legal action being taken against a lab and its employees.
Facilitating Emergency Response
In case of an accident or spill, quick access to MSDS can be life-saving. These sheets contain information such as first-aid measures and spill-handling procedures, which makes a difference in emergency situations.
Promoting Staff Training
MSDS serve as educational tools for new employees. They provide detailed information that can enhance the training programs in your lab. Examples of training programs in labs include chemical safety and handling, emergency response procedures, proper use of personal protective equipment (PPE), and understanding the importance of MSDS. By using MSDS as a resource for training, labs can ensure that all staff members are knowledgeable about potential hazards and how to handle them safely.
Conducting Risk Assessments
Risk assessments are regular parts of lab operations. MSDS provide the necessary data to conduct thorough risk assessments, identify potential hazards, and implement preventive measures.
Buying Chemicals Safely
When you need to buy zinc chloride online or any other chemical, it’s important to have all the necessary safety information, such as hazards associated with the chemical, safe handling procedures, and first-aid measures in case of exposure or ingestion. Trusted suppliers like Post Apple Scientific provide MSDS with their products.
Reducing Environmental Impact
Environmental safety is a growing concern in lab operations. MSDS outline the proper disposal of hazardous chemicals. Following these guidelines can reduce the environmental impact of your lab activities.
Material safety data sheets offer information that helps in handling chemicals safely and complying with UNECE regulations. Regular use of MSDS fosters a culture of safety and enhances overall lab operations. Whenever you need chemicals for your lab, you can contact Post Apple Scientific to fulfill your needs.
-

Tips and Tricks for Cleaning Your Laboratory
Cleanliness is a critical component of safety and accuracy in laboratory management. A clean lab impacts every application you conduct, from ensuring a sterile environment for medical research to maintaining the purity of chemical testing. Here are some expert tips and tricks for cleaning your laboratory, enhancing safety, and maintaining your lab’s high standard of cleanliness.
Develop a Compliant Cleaning Schedule
A laboratory cleaning schedule requires a detailed timetable adhering to industry standards and the specific needs of each lab. The first step to a clean lab is to develop a schedule that aligns with regulatory compliance. Laboratory workers should regularly clean and sanitize their work surfaces and lab equipment after each use to remain safe and compliant. Remember to document all cleaning activities, including which agents you use and any irregular findings, to promote thorough lab bookkeeping.
Use the Appropriate Cleaning Agents
Choosing the right cleaning agents is one of the most critical decisions in laboratory sanitation. Depending on the nature of your research and the materials you’re cleaning, choosing the wrong solvent can lead to contamination or chemical reactions. Acidic solutions might be unsuitable for surfaces susceptible to corrosion, while harsh alkalis could damage glassware. Consider using a neutral detergent or soap for general use and more specific agents like 70% ethanol or hydrochloric acid for specific cleaning applications.
Use Proper Cleaning Tools
The cleaning tools you choose are just as critical as the cleaning agents. You should designate each brush, mop, and cloth for specific uses to prevent cross-contamination. Color coding may be ideal in biological labs—for example, a red mop for contaminated areas and a blue mop for general cleaning. Invest in high-quality microfiber cloths that trap dust better than traditional fabrics. Ensure your staff understands how to disassemble equipment to clean every nook and cranny and use cleaning equipment that can reach those corners, such as manual brushes or autoclaves.
Dispose of Old Chemicals and Samples Properly
Regularly assessing and disposing of expired or unusable chemicals and samples is a pivotal aspect of lab hygiene. These assessments allow your lab staff to create much-needed space and prevent accidental use of deteriorated reagents, which could affect experiment results. Develop a systematic removal protocol for outdated items, ensuring your lab disposes of waste by local, national, and international regulations.
Laboratory cleanliness must be an integral part of the daily workflow. By implementing these tips and tricks for cleaning your laboratory, you can ensure your laboratory is a pristine and safe environment for scientific innovation. Get started on your cleaning protocols by stocking up on the cleaning solvents and materials you need at Post Apple Scientific. Here, you can buy hydrochloric acid and other common laboratory cleaning products. Browse our chemical supplies today to help promote a safe and sanitized lab environment.
-
The Science of Oxalic Acid: Chemical Reactions and Uses
If you are preparing to work with oxalic acid, it is crucial to know the science behind it, its chemical reactions, and its uses. This organic compound has a molecular formula of C₂H₂O₄ and is naturally occurring in many plants, particularly leafy vegetables like spinach. Additionally, oxalic acid can be synthetically produced for industrial purposes. Keep reading to dive into the properties, chemical reactions, applications, and benefits of this fascinating compound.
What Is Oxalic Acid?
Oxalic acid, also known as ethanedioic acid, is a simple dicarboxylic acid. Many experts consider it to be the simplest dicarboxylic acid because it possesses two carboxyl groups. This organic compound exists in two forms: an anhydrous form, which is a colorless crystalline solid, and a dihydrate form, which appears as a white crystal.
Scientists can synthesize oxalic acid in laboratories, but it also naturally occurs in various sources. Plants, fungi, and even some animals can produce oxalic acid as a byproduct. This acid has a wide range of applications, including uses in the textile, pharmaceutical, and metal cleaning industries.
With its unique chemical properties, oxalic acid continues to intrigue researchers and scientists alike, contributing to our understanding of organic compounds and their potential applications.
Properties of Oxalic Acid
Oxalic acid exhibits notable physical and chemical properties:
Solubility
Oxalic acid exhibits high solubility in water, which increases as temperatures rise. However, it shows limited solubility in organic solvents, such as ethanol and acetone, making it predominantly soluble in aqueous environments.
Acidity
Oxalic acid is considered a weak acid because it undergoes partial ionization in aqueous solutions. This process results in the generation of various ions.
Toxicity
Oxalic acid possesses toxic properties for humans due to its affinity for binding with calcium ions in the bloodstream, forming insoluble calcium oxalate crystals. These crystals can cause kidney damage and other health complications. Note that the lethal dose of oxalic acid for humans is around 15–30 grams, highlighting its significant and potent toxicity. When working with oxalic acid and its byproducts in a facility, make sure to label it as a dangerous substance in order to ensure you use it safely.
Oxalic Acid Chemical Reactions
Inducing chemical reactions with oxalic acid is essential for understanding its applications and behavior. Here are some key reactions:
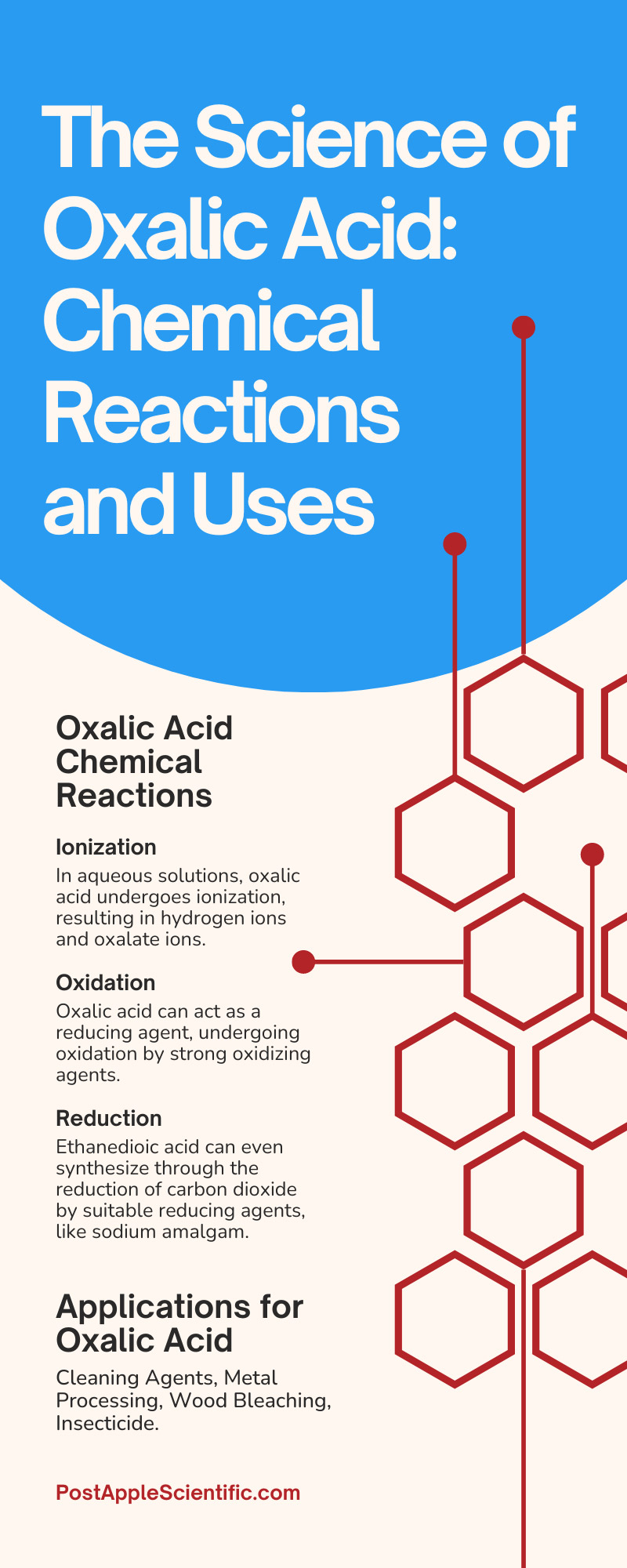
Ionization
In aqueous solutions, oxalic acid undergoes ionization, resulting in hydrogen ions and oxalate ions. Ionization is crucial in many applications, such as the complexation of metal ions and analytical precipitation techniques.
Oxidation
Oxalic acid can act as a reducing agent, undergoing oxidation by strong oxidizing agents. It converts to carbon dioxide (CO₂) and water (H₂O) after oxidizing.
Reduction
Ethanedioic acid can even synthesize through the reduction of carbon dioxide by suitable reducing agents, like sodium amalgam.
Applications for Oxalic Acid
The versatility and unique properties of oxalic acid give rise to unique applications across various industries, including the following:
Cleaning Agents
Due to its acidic nature and exceptional chelating ability, ethanedioic acid is a powerful cleaning agent, particularly for removing stubborn rust or iron stains. Its descaling and decolorizing properties make it effective for restoring the shine of metal surfaces.
Metal Processing
Oxalic acid plays a crucial role in the metallurgical industry, where many experts use it for metal extraction, refining, and electroplating. By forming stable complexes with metal ions, oxalic acid facilitates the separation and purification of metals.
Wood Bleaching
Ethanedioic acid serves as a valuable ingredient in wood bleaching agents. Its unique ability to break down lignin and tannins in wood contributes to lightening the color and enhancing the aesthetic appeal of wooden objects and surfaces.
Insecticide
Oxalic acid exhibits potent insecticidal properties, making it a valuable substance for pest control. Specifically, it wards off pests like mites and aphids, safeguarding plants and promoting healthy growth.
These diverse applications highlight the significance of oxalic acid in various industries, showcasing its immense versatility.
Benefits of Using Oxalic Acid
Oxalic acid also offers several benefits with its applications:
Eco-Friendly
As a naturally occurring compound, ethanedioic acid is biodegradable and does not harm to the environment like many synthetic chemicals. This makes it a sustainable substance for businesses looking to have a neutral impact on the environment. Because we can source oxalic acid from plants, we can consider it renewable and eco-friendly!
Cost-Effective
The widespread availability of oxalic acid in plants and its ease of synthesis make it a cost-effective compound for various industrial processes. Its low production cost and abundance in nature contribute to its affordability, making oxalic acid an attractive substance for businesses aiming to optimize expenses.
Versatile
The ability of oxalic acid to form stable complexes with metal ions gives it a wide range of applications in different industries, like metalworking. Professionals in this field can use oxalic acid as a cleaning agent, rust remover, and even as a reagent in chemical reactions. Its versatility opens up opportunities for innovation and creativity in various fields.
Where To Find Oxalic Acid
If you are looking to take advantage of oxalic acid for its many manufacturing or industrial applications, you might be wondering where to buy oxalic acid. Fortunately, you don’t need to look far when sourcing this compound and many other chemicals and solvents. At Post Apple Scientific, we supply chemicals and other materials for laboratories, manufacturers, and other facilities. Browse our online collection of reagents, including oxalic acid, for your unique applications.
Understanding the science of oxalic acid is vital for taking full advantage of its properties. From its discovery as a naturally occurring compound to its diverse array of synthetic uses, oxalic acid is a remarkably versatile substance in numerous industries. Its distinctive chemical reactions and benefits make it essential for scientific research.
As scientists delve deeper into the complexities of oxalic acid, new insights and applications are likely to emerge, further solidifying this compound’s significance in the realms of chemistry, materials science, and manufacturing. If you are looking for resources, don’t forget to browse Post Apple Scientific for reagents such as oxalic acid to meet your business’s chemical needs.
-
3 Ways To Safely Dispose of Zinc Chloride
Zinc chloride is a crucial compound used in various industries such as electronics, manufacturing, and metallurgy. However, improper disposal of this hazardous substance can lead to severe environmental damage and pose human health risks. Because of these risks, it’s crucial to understand the proper ways to dispose of this substance. Keep reading to discover three ways to dispose of zinc chloride safely.
1. Disposal at a Hazardous Waste Facility
To dispose of zinc chloride safely, take it to a designated hazardous waste facility. These sites handle and process hazardous materials in compliance with environmental regulations.
Before disposal, seal the zinc chloride in a sturdy, leakproof container. Label the container with hazard and chemical information for identification. Don’t forget to wear safety gear such as gloves and goggles during the handling process. Check if the facility accepts hazardous waste, what their drop-off schedule is, and what their fees are. Seek guidance from local authorities or environmental agencies to find licensed hazardous waste facilities.
2. Neutralization Process
Neutralizing zinc chloride is another effective technique for safe disposal. The process involves mixing the waste material with a suitable base, such as sodium hydroxide or sodium carbonate, to create a less harmful product that can be disposed of as nonhazardous waste.
To execute this method, start by wearing appropriate personal protective equipment, including gloves, splash goggles, a lab coat, and an apron. Work in a well-ventilated area with proper safety precautions in place. Carefully combine the base and zinc chloride, continually monitoring the pH until the mixture reaches a neutral range. Once the neutralization occurs, you can then dispose of the resulting solution as regular waste or by flushing it down the drain, as long as permitted by local regulations.
Keep in mind that neutralization is a potentially dangerous procedure and requires a trained professional to ensure safety and compliance with local regulations.
3. Hire Professional Disposal Services
A third option for properly disposing of zinc chloride is to enlist the help of professional hazardous waste disposal services. These experts have the training, equipment, and permits necessary to manage and dispose of hazardous materials like zinc chloride in a safe and compliant manner.
To find a reputable provider, seek recommendations from industry colleagues or consult online resources and directories. Make sure that any chosen contractor is licensed and in good standing with relevant regulating authorities. Always discuss the specifics of the disposal, such as quantity, packaging requirements, and fees, to avoid issues.
It is crucial to recognize the potential hazards associated with zinc chloride and the importance of following these three ways to safely dispose of zinc chloride. By adhering to these methods and safety procedures, you can prevent harm to the environment and people, ensuring a safer and cleaner ecosystem for generations to come. Now that you know how to handle this material safely, you can responsibly purchase zinc chloride for your professional experiments and applications. Browse our selection of zinc chloride and other chemical solutions at Post Apple Scientific today.
-

The Role of Hydrochloric Acid in Industrial Processes
Hydrochloric acid, a clear, highly corrosive mineral acid, is known for its versatility and widespread use in various industrial processes. Soluble in water, it is a crucial ingredient in multiple manufacturing stages across numerous industries. Delve into this exploration of the role of hydrochloric acid in industrial processes, uncovering its significance in food manufacturing, the metal industry, and the oil and ore industries.
Hydrochloric Acid in Food Manufacturing
A primary function of hydrochloric acid in the food industry is regulating acidity levels, which is vital for maintaining appropriate taste and texture. Acting as a pH regulator, hydrochloric acid creates a balanced environment for certain enzymes to work effectively. Food manufacturers also use hydrochloric acid to enhance the flavor of their products by creating a balanced sour taste.
Hydrochloric Acid in the Metal Industry
In the metal industry, hydrochloric acid is indispensable for processes like pickling, a treatment used to remove scale and rust from steel surfaces. During this step, the acid dissolves the impurities, preparing the surface for further processing, such as galvanizing, electroplating, or painting. Hydrochloric acid is ideal for metal processing given its effectiveness and cost-efficiency, significantly reducing the need for mechanical cleaning methods.
Hydrochloric acid’s role in metal processing extends to the cleaning of non-ferrous metals like copper, brass, and aluminum. Its high solubility in water and powerful corrosive properties make it an effective solution to remove tarnish and oxidation. Manufacturers also use it in the synthesis of metal salts and for extracting precious metals like gold and platinum.
Hydrochloric Acid in the Oil and Ore Industries
The oil and ore industries rely heavily on hydrochloric acid for mineral and resource extraction. Professionals injecthydrochloric acid solutions into rock formations to dissolve minerals and increase the reservoir’s permeability when extracting crude oil. This method, known as acidizing, boosts oil production by creating channels for the oil to flow more efficiently.
Similarly, hydrochloric acid plays a critical role in the extraction of valuable ores. It can help dissolve the impurities in the metal ores, separating the targeted metal from waste material. This process results in the ore’s concentrated and purified form, suitable for further refining and processing.
The role of hydrochloric acid in industrial processes is indispensable. Its widespread use in food manufacturing, the metal industry, and the oil and ore industries highlights its versatility and effectiveness across diverse sectors. Understanding the significance of this powerful chemical compound allows us to appreciate its numerous contributions to everyday products and processes. You can find bulk solutions at Post Apple Scientific if you’re a business owner looking for hydrochloric acid solutions for any of these applications and more.
-

3 Effects of Ethyl Alcohol on the Human Body
Ethyl alcohol, also known as ethanol, is a colorless, flammable liquid with various applications. It is present in everyday items, from the alcohol we consume in beverages to household cleaning products and even fuel. Because it is so common in daily products, the effects of ethyl alcohol on the human body have long been the subject of extensive scientific research. Keep reading to learn more about these effects and how to minimize associated risks.
Skin and Respiratory Irritation
One immediate effect of ethyl alcohol on the human body is skin and respiratory irritation. Ethyl alcohol in high concentrations can result in skin inflammation. Furthermore, inhaling ethanol vapor can irritate the nasal passages, throat, and respiratory system. These high-concentration vapors and their irritating effects can have an impact on your lungs and strain breathing. Professionals and manufacturers working in close contact with ethyl alcohol are at greater risk of experiencing such effects.
Sleepiness or Stupor
In addition to irritation, ethyl alcohol can cause sleepiness and stupor. When consumed, even in moderate amounts, ethanol can have a sedative effect on the body. Excessive consumption or direct intake of this chemical can lead to extreme drowsiness, confusion, and unconsciousness. In more severe cases, acute ethyl alcohol poisoning can lead to coma or death. As we are all aware, consuming too much alcohol in the form of beverages can be extremely hazardous to one’s health.
Impaired Perception and Coordination
Impaired perception and coordination are further examples of the detriments of ethyl alcohol on the human body. Ethanol consumption is known to affect balance and motor control. Alcohol directly impacts judgment, reaction time, and concentration. This impairment plays a significant role in the increased risk of injury and accidents when people are under the influence of ethanol, such as in motor vehicle collisions.
Avoiding Ethyl Alcohol Exposure
Avoiding ethyl alcohol exposure is critical to our overall health and well-being. Wearing appropriate personal protective equipment, such as gloves and respiratory masks, can prevent direct contact with ethanol for those who work with it professionally. In our daily lives, limiting consumption and not abusing alcohol can help reduce the negative impact of ethyl alcohol on our perception, coordination, and long-term health. Furthermore, using alternative cleaning solutions without ethyl alcohol can minimize the risk of skin and respiratory irritation.
These effects of ethyl alcohol on the human body are multifaceted. By understanding these effects and taking precautions to minimize exposure, we can contribute to our overall health and well-being. And if you’re wondering where to buy ethyl alcohol from a trustworthy source, turn to Post Apple Scientific. We offer bulk ethyl alcohol orders for laboratories, manufacturers, and other industries that use ethyl alcohol in large quantities.
-

Sulfuric Acid Solutions and Their Role in Water Treatment
Sulfuric acid is often known for its corrosive properties, but it plays an essential role in various industrial processes. Among these applications, one of the most important is its use in water treatment. Learn more about the properties of sulfuric acid solutions and their role in water treatment while also exploring the environmental benefits and necessary safety precautions associated with the use of this material.
Understanding Sulfuric Acid Solutions
Sulfuric acid (H2SO4) is a strong, highly corrosive, and oily liquid that can be colorless or slightly yellow. It has the unique ability to dissolve many substances, making it an effective cleaning and neutralizing agent. The chemical composition of sulfuric acid allows it to react with various impurities present in water, enabling the efficient removal of pollutants. The industrial applications of sulfuric acid have evolved over time and include the many water treatment aspects we see today.
The Role of Sulfuric Acid in Water Treatment
One of the critical applications of sulfuric acid in water treatment is the pH adjustment of water. Neutralizing alkaline substances such as calcium carbonate in wastewater is crucial in various industrial processes. Sulfuric acid solutions react with alkaline contaminants, forming a precipitate that attendants can easily separate from the water. This reduces the harmful effects of these contaminants and allows for easier implementation of secondary treatments for thorough water purification.
Benefits of Using Sulfuric Acid in Water Treatment
There are several benefits of using sulfuric acid in water treatment, including environmental benefits. By treating and purifying water using sulfuric acid solutions, industries can ensure the safe disposal of wastewater and the reduction of environmental pollution. In an era where water scarcity is a significant concern and water pollution is ever-increasing, it is essential to ensure effective water treatment processes are in place to maintain public health and safety.
When Using Sulfuric Acid
When using sulfuric acid solutions in water treatment, it is vital to adhere to all necessary safety precautions. Proper handling, storage, and use of sulfuric acid must be enforced to minimize the risk of accidents and chemical exposure. Protective equipment such as goggles and chemical-resistant gloves should be used. Additionally, storage and handling areas must have an appropriate ventilation system.
Sulfuric acid solutions and their roles in water treatment are indispensable for the safe and efficient purification of water in industrial and municipal applications. By using sulfuric acid solutions to adjust pH levels and minimize contaminants, industries can reduce the harmful effects of water pollution and contribute to a cleaner, safer environment. If you’re looking into these solutions for your own water treatment facility, you can find bulk sulfuric acid solutions at Post Apple Scientific. Learn more about our bulk chemical shipping options today.
-

Caffeine as a Lab Reagent: Uses and Precautions
The average consumer might think of coffee, tea, and energy drinks when they think of the word “caffeine.” However, specific types of caffeine have important applications in scientific research and laboratory studies. Scientists use lab-grade caffeine as a lab reagent as it plays a significant role in several research areas. Keep reading to explore caffeine as a lab reagent, its uses, and precautions, and why it’s essential to scientific advancements.
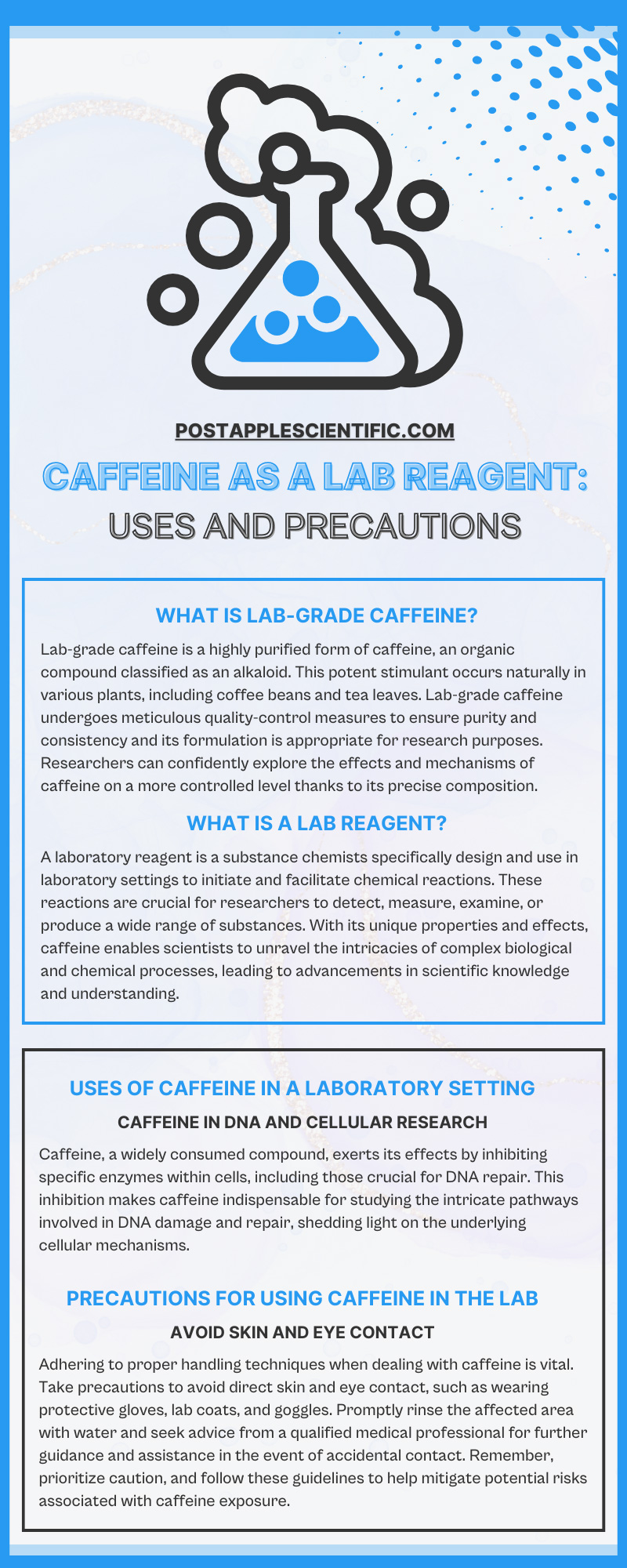
What Is Lab-Grade Caffeine?
Lab-grade caffeine is a highly purified form of caffeine, an organic compound classified as an alkaloid. This potent stimulant occurs naturally in various plants, including coffee beans and tea leaves. Lab-grade caffeine undergoes meticulous quality-control measures to ensure purity and consistency and its formulation is appropriate for research purposes. Researchers can confidently explore the effects and mechanisms of caffeine on a more controlled level thanks to its precise composition.
What Is a Lab Reagent?
A laboratory reagent is a substance chemists specifically design and use in laboratory settings to initiate and facilitate chemical reactions. These reactions are crucial for researchers to detect, measure, examine, or produce a wide range of substances. With its unique properties and effects, caffeine enables scientists to unravel the intricacies of complex biological and chemical processes, leading to advancements in scientific knowledge and understanding.
Uses of Caffeine in a Laboratory Setting
Caffeine is a versatile lab reagent with numerous applications across various scientific disciplines. Scientists use it widely in DNA and cellular research, where its stimulating properties help them investigate various molecular processes. Additionally, caffeine aids in pharmacological studies, where it serves as a valuable tool scientists can use to explore the effects of certain compounds on cellular activity. With its multifaceted nature, caffeine continues to contribute to advancements and discoveries in diverse areas of scientific inquiry. Learn more about each application below.
Caffeine in DNA and Cellular Research
Caffeine, a widely consumed compound, exerts its effects by inhibiting specific enzymes within cells, including those crucial for DNA repair. This inhibition makes caffeine indispensable for studying the intricate pathways involved in DNA damage and repair, shedding light on the underlying cellular mechanisms. Furthermore, this reagent’s impact on various cellular processes also aids in cancer research and exploration of the interactions between caffeine and various bodily processes.
Caffeine in Pharmacological Studies
The pharmacy industry also uses caffeine widely as a key investigative component when researchers study the effects of a specific medication on various body systems. Because caffeine is a common ingredient in many consumer products, many researchers desire to understand its effects. Notably, researchers delve into the intricate impact of caffeine on the cardiovascular system, the central nervous system, and metabolic processes. By comprehending these effects in greater detail, scientists can harness the properties of caffeine to develop and optimize pharmaceuticals and enhance medicine delivery systems for improved therapeutic outcomes.
Precautions for Using Caffeine in the Lab
Although caffeine is generally safe to use, it is important to take several precautions when working with caffeine as a lab reagent to ensure the safety of lab personnel. These safety precautions must include wearing appropriate personal protective equipment, following proper handling procedures, and implementing safety measures to prevent accidents or exposure. By being diligent with these precautions, scientists can create a safer working environment and mitigate potential risks associated with caffeine in the laboratory setting.
Avoid Skin and Eye Contact
Adhering to proper handling techniques when dealing with caffeine is vital. Take precautions to avoid direct skin and eye contact, such as wearing protective gloves, lab coats, and goggles. Promptly rinse the affected area with water and seek advice from a qualified medical professional for further guidance and assistance in the event of accidental contact. Remember, prioritize caution, and follow these guidelines to help mitigate potential risks associated with caffeine exposure.
Prevent Ingestion
Lab-grade caffeine has a higher concentration than its consumer-grade counterpart, with the potential for harmful or even fatal effects if anyone were to ingest it. Establish strict handling protocols to ensure the safety of lab personnel. Implement designated areas for eating and drinking, which should remain separate from lab spaces. Additionally, label all lab-grade caffeine-containing substances clearly and prominently to minimize the risk of unintended consumption. Make sure your staff understands the common symptoms of accidental anhydrous caffeine ingestion, such as elevated heart rate, nausea, anxiety, dizziness, and more. By adopting these measures, we can prioritize the well-being and safety of everyone in the laboratory environment.
Proper Caffeine Storage and Ventilation
Having a designated area specifically for storing reagent caffeine is crucial. Keep this area separate from incompatible reagents and heat sources to prevent potential reactions or degradation. Additionally, maintaining a dry and well-ventilated storage environment is also critical, as lab-grade caffeine is hygroscopic and can degrade under humid conditions. By paying attention to these details, you can ensure the longevity and quality of your caffeine supply.
Where To Obtain Lab-Grade Caffeine
You can find a range of products at Post Apple Scientific if you oversee a pharmaceutical laboratory or other research-related lab looking for reagents for your studies. We offer bulk caffeine anhydrous powder for studies and experiments if you need this particular material. You can also find a range of other reagents, chemical solutions, cleaning solutions, and more for professional and educational lab use. Feel free to browse our collection of reagents and other lab equipment to find all the supplies your laboratory needs in one place.
It is of the utmost importance for laboratory personnel to understand the various aspects of caffeine as a lab reagent, including its uses, precautions, and potential risks. By having a comprehensive understanding, they can effectively safeguard themselves and maintain the integrity of their research endeavors. Handling lab-grade caffeine with meticulous care is important, as doing so promotes scientific advancements and ensures the safety of those dedicated to exploring the wonders of this exciting field. By exercising caution and adhering to proper usage protocols, researchers can unlock new possibilities and uncover valuable insights while minimizing potential hazards. Learn more today at Post Apple Scientific if you have any questions about obtaining and using lab-grade caffeine. Don’t forget to browse our full listings for lab-grade caffeine and other helpful laboratory regents today.
-

The Role of Nitric Acid Solutions in Metal Etching
Understanding the role of nitric acid solutions in metal etching is crucial for metal finishing companies, foundries, and manufacturing companies. To grasp the significance of nitric acid in this process, you must first learn the basics of nitric acid and why it is so effective in etching metals.
Understanding Nitric Acid Solutions
Nitric acid (HNO3) is a highly corrosive and powerful oxidizing agent that serves many purposes in various industries. Its properties make it ideal for etching metals, which is a technique to engrave, shape, or alter the surface of metals. Metal etching involves removing a layer of metal to create a design or pattern on the surface. Nitric acid is well-suited for this application due to its ability to react with different metals, dissolving them and producing a stable oxide layer.
Uses of Nitric Acid for Metal Etching
One of the primary uses of nitric acid for metal etching is creating intricate designs on metal. Precision and control are essential for manufacturers of electronics, medical devices, and automotive parts. For instance, professionals in the aerospace industry rely on HNO3 to create unique identification codes on metal parts or produce surface patterns for enhanced bonding. Jewelers and manufacturers even use nitric acid on precious metals such as silver!
Benefits of Using Nitric Acid
One key benefit of using nitric acid is its ability to preserve the integrity and surface properties of the etched object. This is beneficial for applications where maintaining the metal’s original characteristics, such as corrosion resistance or mechanical strength, is paramount.
Nitric acid plays an indispensable role in metal etching across various industries. Its abilities to dissolve metals, create intricate designs, and preserve the original properties of the etched surface make it exceptional for professional metal etching. If you’re looking for nitric acid solutions for your manufacturing or metalworking business, browse our selection at Post Apple Scientific. We provide bulk solvents and solutions for a wide range of applications.
-

Best Practices for Handling and Storing Solvents
Solvents play a crucial role in many processes across various industries. A solvent is a liquid substance that can dissolve other substances, forming a solution. While their properties make them indispensable, mishandling solvents can pose significant risks. Therefore, adhering to the best practices for handling and storing solvents is imperative to ensure a safe work environment. Keep reading to learn the essential guidelines and discuss the importance of maintaining caution while working with these substances.
Label Each Solvent
The first step in ensuring safety when dealing with solvents is properly labeling each container. Labels should prominently display the solvent’s name, hazard warnings, and safety precautions. Additionally, it’s essential to abide by regional and national regulations regarding labeling hazardous substances.
Choose a Properly Ventilated Storage Area
Selecting a suitable storage area is another critical aspect of responsible handling. You must store solvents in a well-ventilated space to prevent the buildup of harmful fumes. Additionally, if your laboratory or other facility contains hazardous substances, such as flammable materials, you should store these separately in a designated storage space. Put them in fire-resistant cabinets away from ignition sources. Consider safety requirements and segregate solvents based on compatibility to avoid dangerous reactions.
Always Use Personal Protective Equipment
When you’re working with solvents, using personal protective equipment (PPE) is non-negotiable. PPE may include gloves, safety goggles, face masks, or lab coats, depending on the type and concentration of the solvent. Select PPE based on the material’s resistance to the specific solvent in use, ensuring complete protection for the wearer.
Practice Safe Handling Techniques
Practicing safe handling techniques minimizes the risk of accidents or harm. Never pour solvents directly over a container. Instead, use a funnel to reduce spillage. Do not eat, drink, or smoke while working with solvents, as this increases the chance of ingestion or ignition. Always wash your hands thoroughly after handling solvents.
Proper education and training on best practices for handling and storing solvents play crucial roles in ensuring a safe workplace. By following these guidelines, businesses can mitigate the risks and dangers of working with solvents. If you’re looking for a supplier who offers bulk solvents and all the proper care and handling information, reach out to Post Apple Scientific. Our team of experts is happy to answer any safety or handling questions for common solvents you need.