Your cart is currently empty!
Author: Kate Romeo
-

How Nitric Acid Plays a Role in Metal Passivation
If you’re looking to preserve and protect metal surfaces, passivation is the way to go. This process enhances corrosion resistance, ensuring that metal surfaces maintain their appearance and functionality over time. One vital component in this procedure is nitric acid. Read on to find out how nitric acid plays a role in metal passivation.
The Passivation Process
Passivation is a chemical process that causes a thin, protective layer to form on the surface of metals. This layer, typically composed of metal oxide or other corrosion-resistant compounds, shields the metal from further reactions with the environment. Nitric acid is often used as an oxidizing agent in this process, speeding up the formation of the protective oxide layer on the metal surface.
Nitric Acid and Stainless Steel
One common application of nitric acid in passivation is on stainless steel. Stainless steel is well known for its resistance to rust and corrosion due to the presence of chromium. When nitric acid is applied to stainless steel, it reacts with the chromium to form a chromium oxide layer. This transparent, passive film protects the underlying metal from further oxidation and corrosion.
Nitric Acid and Aluminum
Another example of nitric acid’s role in passivation is with aluminum. Upon contact with nitric acid, aluminum develops an aluminum oxide layer on its surface. This film is not only corrosion-resistant, but also provides a base for the application of additional protective coatings, such as paints or other sealants.
Benefits of Nitric Acid in Passivation
Nitric acid offers several advantages in the passivation process. Compared to other acids, nitric acid works more quickly to form the passive layer, leading to shorter treatment times and savings on resources. Additionally, nitric acid effectively removes iron contaminants from the surface of stainless steel. By eliminating these impurities, passivation with nitric acid ensures a clean, uniform surface that is more resistant to corrosion.
Now that you know how nitric acid plays a role in metal passivation, find nitric acid for sale online from Post Apple Scientific for all your metal protection needs.
-

The Dos and Don’ts of Neutralizing a Chemical Spill
When working with chemicals, safety is always a top priority. Unfortunately, accidents do happen, and chemical spills are a potential risk in any environment where these substances are stored or used. Knowing how to properly handle and neutralize a chemical spill is essential to maintaining a safe workplace. With that, discover the dos and don’ts of neutralizing a chemical spill.
Do: Assess the Situation
Before attempting to neutralize a chemical spill, it’s crucial to evaluate the situation. Identify the chemical involved, its hazards, and the extent of the spill. Consult the Material Safety Data Sheet (MSDS) or other safety resources for specific information about the chemical and proper handling procedures.
Don’t: Rush In
Never approach a chemical spill without the appropriate personal protective equipment (PPE). Depending on the chemical involved, this may include gloves, goggles, a face shield, and a chemical-resistant apron or suit. Ensure that all personnel involved in the spill response are properly trained and equipped.
Do: Contain the Spill
Once you’ve assessed the situation and donned the appropriate PPE, work to contain the spill. Use absorbent materials like spill pads to prevent the spill from spreading. If necessary, construct barriers to keep the spill away from drains or other sensitive areas.
Don’t: Use Incompatible Neutralizing Agents
It’s important to use the correct neutralizing agent for the specific chemical involved in the spill. Using an incompatible agent can lead to dangerous reactions or make the situation worse. Always consult safety resources for guidance on the appropriate neutralizing agent.
Do: Clean Up Safely
After neutralizing the spill, carefully clean up the area. Collect the neutralized material and any absorbent materials used in the containment process. Properly dispose of the waste according to local regulations and your organization’s waste management protocols. At Post Apple Scientific, we have certain solvents for sale that may help clean up chemical spills in your laboratory.
Now that you know the dos and don’ts of neutralizing a chemical spill, put in the effort to make your lab a safer place for all your employees.
-
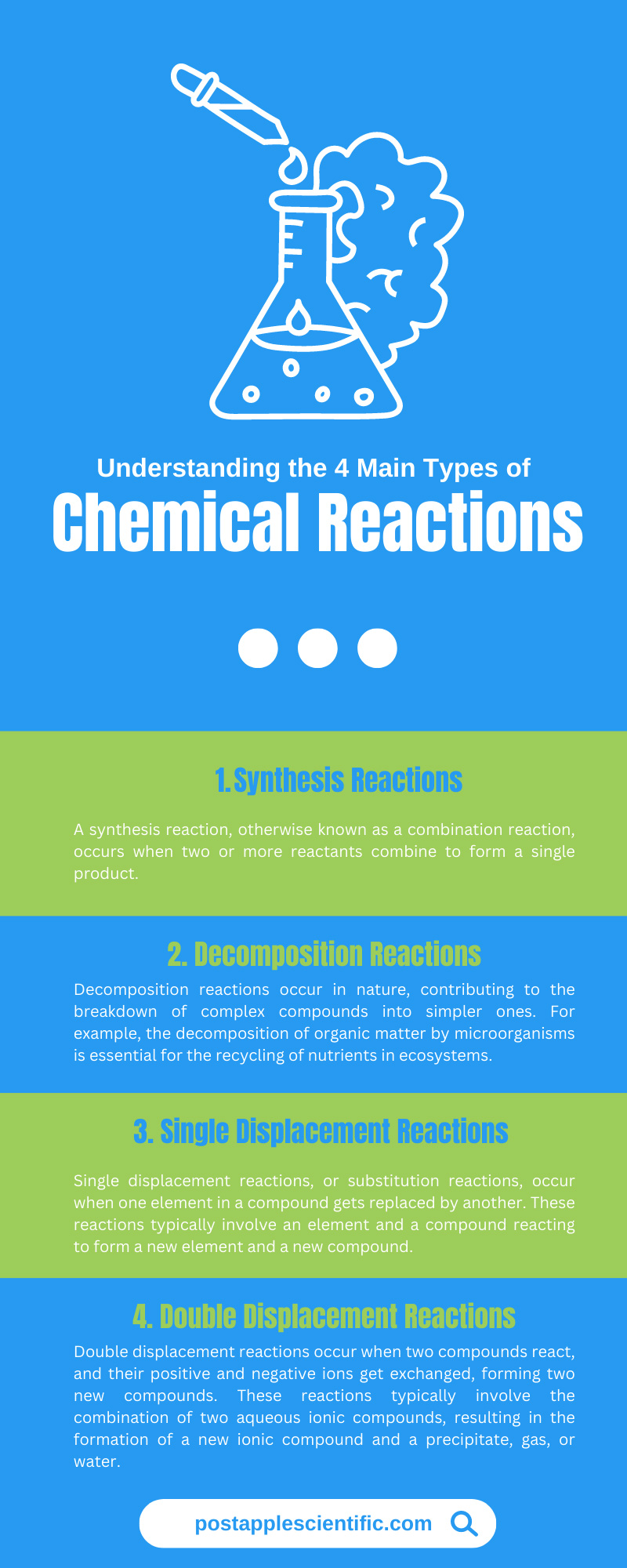
Understanding the 4 Main Types of Chemical Reactions
Chemical reactions are essential to the world around us, from the simplest processes in nature to the most complex industrial applications. There are countless types of chemical reactions, but they can get classified into four main categories: synthesis, decomposition, single displacement, and double displacement reactions. Read on for an understanding of the four main types of chemical reactions.
Synthesis Reactions (Combination Reactions)
A synthesis reaction, otherwise known as a combination reaction, occurs when two or more reactants combine to form a single product. These reactions are typically represented by the following general form:
A + B → AB
Synthesis reactions are typically exothermic, meaning they release energy in the form of heat. These reactions are crucial in the formation of new compounds and play a significant role in various natural processes and industrial applications.
One of the most recognizable synthesis reactions is photosynthesis. In this process, plants convert carbon dioxide and water into glucose and oxygen using sunlight as an energy source.
Examples of Synthesis Reactions
Let’s look at a few common synthesis reactions to give you a better understanding.
Formation of Water
2H₂(g) + O₂(g) → 2H₂O(g)
In this example, hydrogen gas (H₂) and oxygen gas (O₂) combine to form water (H₂O). This reaction is a vital component of combustion processes, such as the burning of hydrogen fuel.
Formation of Magnesium Oxide
2Mg(s) + O₂(g) → 2MgO(s)
When magnesium metal (Mg) gets heated in the presence of oxygen gas (O₂), it forms magnesium oxide (MgO), a white solid. This reaction is an example of a metal reacting with a non-metal to form an oxide.
Formation of Sodium Chloride
2Na(s) + Cl₂(g) → 2NaCl(s)
In this reaction, sodium (Na) combines with chlorine gas (Cl₂) to produce sodium chloride (NaCl), also known as table salt. The reaction is highly exothermic and illustrates the formation of an ionic compound from its constituent elements—it also makes food taste good!
Decomposition Reactions
Decomposition reactions fall on the other end of the spectrum from synthesis reactions. In a decomposition reaction, a single compound separates into two or more simpler products. These reactions often require an input of energy, such as heat, light, or electricity, and are generally endothermic, meaning they absorb energy. You can represent decomposition reactions with the following general form:
AB → A + B
Decomposition reactions occur in nature, contributing to the breakdown of complex compounds into simpler ones. For example, the decomposition of organic matter by microorganisms is essential for the recycling of nutrients in ecosystems.
Examples of Decomposition Reactions
Read on and learn a few types of decomposition reactions.
Electrolysis of Water
2H₂O(l) → 2H₂(g) + O₂(g)
In this example, water (H₂O) decomposes into hydrogen gas (H₂) and oxygen gas (O₂) when an electric current passes through it. This process, known as electrolysis, produces hydrogen for various industrial applications.
Decomposition of Calcium Carbonate
CaCO₃(s) → CaO(s) + CO₂(g)
When calcium carbonate (CaCO₃), commonly found in limestone and marble, gets heated, it decomposes into calcium oxide (CaO), also known as quicklime, and carbon dioxide gas (CO₂). This reaction is essential in the production of cement and lime.
Thermal Decomposition of Potassium Chlorate
2KClO₃(s) → 2KCl(s) + 3O₂(g)
When potassium chlorate (KClO₃) gets heated, it breaks down into potassium chloride (KCl) and oxygen gas (O₂). This reaction gets used as a source of oxygen in emergency oxygen generators and as an oxidizer in fireworks and explosives.
Single Displacement Reactions (Substitution Reactions)
Single displacement reactions, or substitution reactions, occur when one element in a compound gets replaced by another. These reactions typically involve an element and a compound reacting to form a new element and a new compound. The following general form represents single displacement reactions:
A + BC → AC + B
You’ll find single displacement reactions in nature, too—corrosion and the formation of minerals are processes containing single displacement reactions.
Examples of Single Displacement Reactions
Here are a few instances of single displacement reactions.
Reaction of Zinc With Hydrochloric Acid
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
In this example, zinc metal (Zn) reacts with hydrochloric acid (HCl) to produce zinc chloride (ZnCl₂) and hydrogen gas (H₂). The zinc displaces the hydrogen in the acid, forming a new compound and releasing hydrogen gas.
Reaction of Copper With Silver Nitrate
Cu(s) + 2AgNO₃(aq) → Cu(NO₃)₂(aq) + 2Ag(s)
When copper metal (Cu) gets placed in a solution of silver nitrate (AgNO₃), it reacts to form copper nitrate (Cu(NO₃)₂) and silver metal (Ag). In this reaction, copper displaces silver in the silver nitrate solution, producing solid silver and a new compound.
Double Displacement Reactions (Metathesis Reactions)
Double displacement reactions occur when two compounds react, and their positive and negative ions get exchanged, forming two new compounds. These reactions typically involve the combination of two aqueous ionic compounds, resulting in the formation of a new ionic compound and a precipitate, gas, or water. You can represent double displacement reactions with the following general form:
AB + CD → AD + CB
You can find a natural example of double displacement reactions when acids or bases get neutralized in an environment.
Examples of Double Displacement Reactions
Finally, here are a few examples of this reaction.
Reaction of Silver Nitrate and Sodium Chloride
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
In this example, silver nitrate (AgNO₃) reacts with sodium chloride (NaCl) to produce silver chloride (AgCl) and sodium nitrate (NaNO₃). The silver and sodium ions switch places, forming a precipitate of silver chloride and an aqueous solution of sodium nitrate.
Reaction of Sodium Carbonate and Calcium Chloride
Na₂CO₃(aq) + CaCl₂(aq) → 2NaCl(aq) + CaCO₃(s)
In this reaction, sodium carbonate (Na₂CO₃) reacts with calcium chloride (CaCl₂) to form sodium chloride (NaCl) and calcium carbonate (CaCO₃). The sodium and calcium ions exchange places, resulting in a new aqueous solution and a precipitate of calcium carbonate.
No matter what type of reaction you’re trying to create in your laboratory, Post Apple Scientific can help with our in-stock lab chemicals. Get all the chemicals and lab equipment you need from us—you won’t be disappointed.
Now that you have an understanding of the four main types of chemical reactions, we hope you possess a more in-depth sense of the incredible potential of chemistry. With this knowledge and our chemicals and equipment, you can create incredible reactions in your lab!
-

4 Benefits of Critical Reagent Characterization
An assay is a test to determine whether a particular substance is present in a sample, and it can help you determine the amount of a substance in a sample. Critical reagent characterization is an essential part of the assaying process. Read these four benefits of critical reagent characterization.
Improved Assay Performance
The impact on assay performance is a significant benefit of critical reagent characterization. By fully understanding the properties and behavior of critical reagents, laboratories can optimize assay conditions and minimize potential sources of variability. This leads to improved accuracy, precision, and performance of the assays, enabling reliable and consistent results.
Regulatory Compliance
Regulatory bodies, such as the FDA, require thorough characterization of critical reagents to validate bioanalytical methods. Labs can demonstrate compliance with regulatory requirements and ensure their assays meet the necessary standards by conducting comprehensive critical reagent characterization. This is essential to gain approval for new drugs and maintain the integrity of the testing process.
Greater Consistency
Characterizing critical reagents ensures consistency across different lots of the same reagent. By establishing a clear set of specifications and acceptance criteria, laboratories can identify and control potential sources of variability between lots. Maintaining the reliability of assays over time and ensuring accurate and reproducible results is essential—critical reagent characterization makes that possible.
Reduced Risk of Assay Failures
The characterization of critical reagents reduces the risk of assay failures that arise from unforeseen issues with reagent quality or performance. By identifying potential problems before they affect the assay, lab workers can minimize the need for retesting and avoid associated costs and delays. This is particularly important in the pharmaceutical industry, where assay failures can negatively affect drug development timelines and costs.
Now that you know these four benefits of critical reagent characterization, work with Post Apple Scientific to buy reagents for assays and other tests.
-

The Importance of Proper Chemical Waste Management for Labs
Chemical waste management is a critical aspect of laboratory operations. Proper handling, storage, and disposal of chemical waste are essential to ensure the safety of laboratory personnel, protect the environment, and maintain regulatory compliance. Read on to learn the importance of proper chemical waste management for labs.
Lab Personnel Safety
While wholesale chemicals are critical for use in laboratories, they can be hazardous to the health and safety of workers in your lab. Exposure to toxic chemicals, corrosive substances, or flammable materials can cause severe injuries or illnesses. Proper chemical waste management minimizes the risk of accidental exposure and helps maintain a safe working environment for laboratory personnel.
We suggest that you ensure all lab personnel have proper training in chemical waste handling and disposal procedures as a baseline. In addition, be sure to provide appropriate personal protective equipment for handling chemical waste.
Environmental Protection
Improper chemical waste disposal can lead to environmental contamination, harming ecosystems and posing risks to human health. Proper chemical waste management practices help prevent pollution of air, soil, and water resources, contributing to a cleaner and healthier environment.
It is essential that you segregate waste based on its properties—don’t mix flammable, corrosive, or toxic products together. In addition, always use clearly labeled containers to transport or store any chemical waste.
Regulatory Compliance
Laboratories must comply with local, state, and federal regulations regarding waste management. Proper waste management practices help labs adhere to these requirements, which is essential for avoiding fines, penalties, and closures.
Be sure to familiarize yourself with the regulations for your area, then develop and implement a waste management plan that meets the requirements. Don’t forget to keep records of chemical waste generation, storage, and disposal so you have proper documentation in case an agency decides to check.
Now that you understand the importance of proper chemical waste management for labs, keep your facility safe and do your part to protect the environment.